In his new career as a Daily Mail columnist, Boris Johnson has a fraction of the responsibility placed upon his shoulders as British prime minister during the Covid pandemic. Nevertheless, in his very first column for the paper he managed to recommend a “wonder drug” appetite suppressant without warning his readers that it is being investigated by the Food and Drug Administration and the European Union for association with suicidal thoughts.
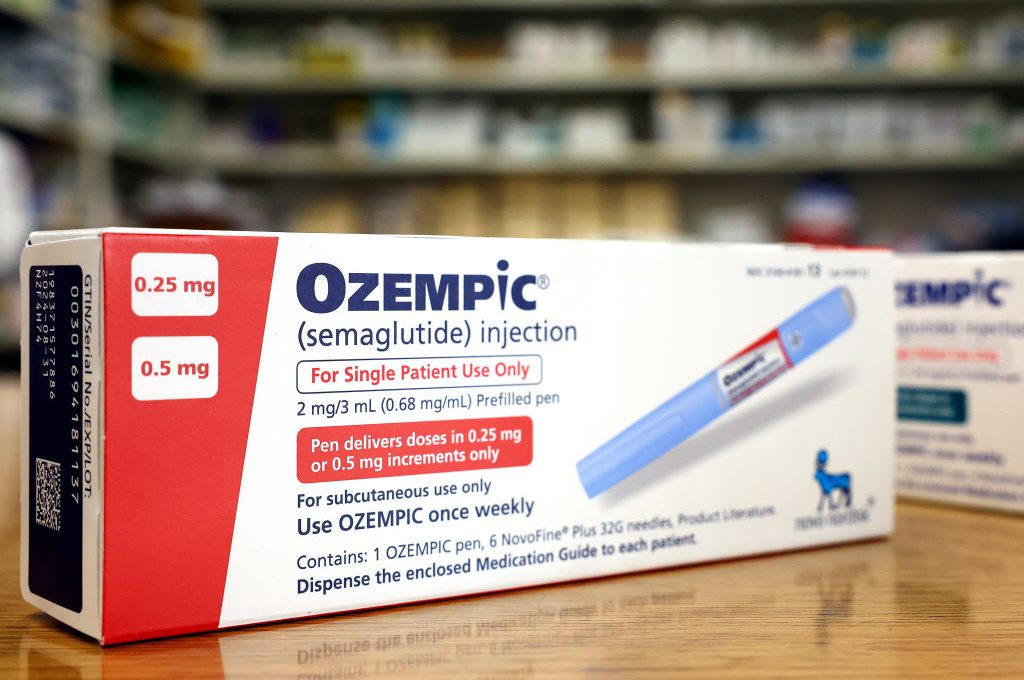
Johnson’s column, published on June 17, recalled how he had been surprised by sudden weight loss in three of his former cabinet colleagues. The answer, he discovered from one of them, was a drug called semaglutide, marketed under the proprietary name Ozempic. The former PM says he then visited a doctor who gave him a prescription for the drug, injecting it into his abdomen once a week in the hope that it would stop his midnight raids of the fridge for cheese and chorizo.
Initially, he wrote, the drug helped him lose four or five pounds a week — at the cost, that is, of making him feel ill. He stopped after a few weeks. Yet that didn’t stop him recommending the drug to other people with great zeal. “I see nothing morally wrong in using these drugs to help you lose weight, any more than it is wrong to use an electrically-assisted bicycle to get up the hill,’ he wrote. “Even for us fatties, it turns out, there is such a thing as satiety — and science has found it.”
Perhaps, but what science has managed to create is a class of drug which has been linked with too rather too many unpleasant — and in some cases fatal — side effects. There is a long history of “wonder” drugs which in the event proved to cause more problems than they were worth. Seven years ago we published a review into twenty-five weight loss drugs which had been approved by regulatory agencies between 1994 and 2003. Every one of the drugs was subsequently withdrawn, mainly because of psychiatric and/or cardiovascular adverse reactions. Deaths were reportedly associated with seven of them.
Following the withdrawal of these products the industry moved on to develop different classes of weight loss drugs: naltrexone-bupropion, phentermine-topiramate and lorcaserin. Yet they, too, proved to be more trouble than they were worth. In a second review we showed that the benefit-harm profile of these new drugs was no different from the previously-withdrawn ones. While they proved effective at helping many people to lose weight it came at the cost of an unacceptable level of adverse side effects, including nausea, vomiting, dizziness, tremors and high blood pressure. In the case of one of the drugs, adverse effects were reported in one in every eleven people who took it. After we published our review, Lorcaserin was withdrawn from the market owing to an increased risk of cancer.
The industry has now moved on to a third class of drugs, known as GLP-1 receptor agonists for weight loss — which include Ozempic (semaglutide) as well as another proprietary product Saxenda (which is a drug called liraglutide). While evidence suggests that these drugs may promote weight loss, the potential harms of using such products are not extensively reported, nor even investigated in Britain. There are hardly any post-marketing trials investigating their possible disadvantages — although we do know that the FDA has received sixty reports of suicidal thoughts among patients on semaglutide and seventy among those on liraglutide.
We’re waiting to see what happens next, but we don’t have much faith in the ability of our regulatory system to assess harms in new drugs. History suggests that today’s “wonder” drugs for fighting obesity may go the way of those which preceded them.
This article was originally published on The Spectator’s UK website.